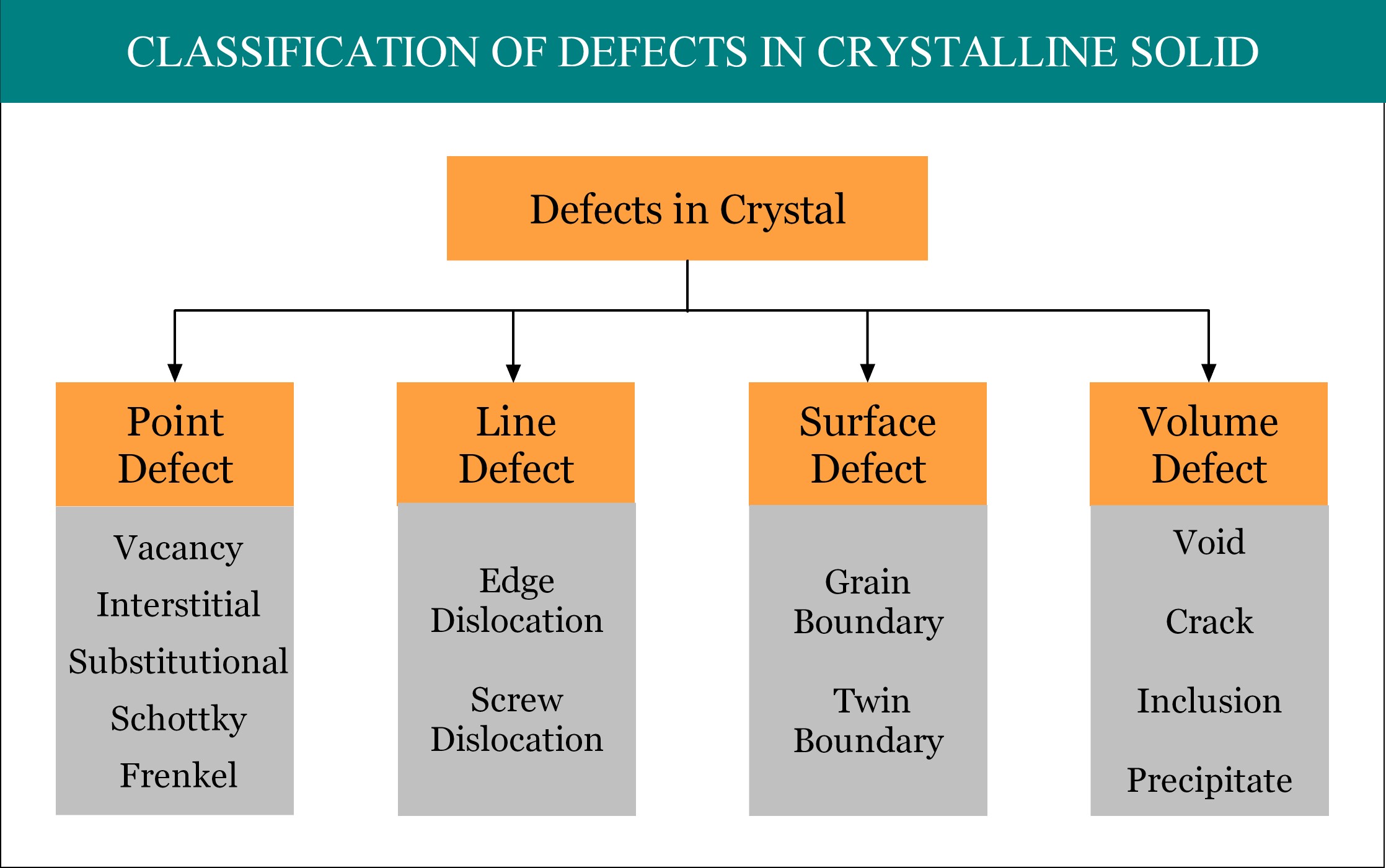
Imperfections or defects in crystalline solid can be broadly classified into four groups, namely, point defect, line defect, surface defect and volume defect. Point defect is considered as the zero dimensional (0-D) defect, as by mathematical definition, a point is unit-less dimensionless quantity! By the way, point defect is the smallest possible defect in any material.
Definition of Point Defect:
A point defect occurs when one or more atoms of a crystalline solid leave their original lattice site and/or foreign atoms occupy the interstitial position of the crystal. So there exist three possibilities by which point defect may occur, as provided below:
- One or more original atoms of the crystal are missing from their corresponding lattice site.
- One or more original atoms are shifted from the original lattice site to the interstitial position in same crystal.
- One or more foreign atoms occupied the interstitial position of the crystalline solid.
- One or more foreign atoms replaced the original atom of the crystal and subsequently occupied the interstitial position.
It is practically impossible to obtain any substance in 100% pure form. Some impurities will always present in it.
Features of Point Defect in Crystalline Solid:
- Position of the point defects may be random or an arranged manner.
- Weight and density of the crystalline solid may increase, decrease or even remain constant for Point defect.
- Point defects are inherent to the natural material, whose temperature is above absolute zero temperature (0K). However, perfect crystal can be obtained in scientific laboratories with tight control of environment.
- Point defect can affect the equilibrium of neighboring atoms and can put them under tension or compression.
- Point defects can change the physical and chemical properties of the solid, such as it can convert an insulator to electrically conductor one, it can increase the hardness of the material.
- Sometime point defects are intentionally created in the solid in a controlled way in order to modify the properties of the material for better functionality.
Causes of Point Defect in Crystalline Solid:
- Diffusion (solid-solid).
- Increase or decrease in temperature in presence of other medium.
- Plastic deformation.
- Particle or ion bombarding, such as sputtering or ion irradiation.
- Deliberate alloying to enhance properties.
- Presence of residual stress.
Types of Point Defect in Crystalline Solid:
Point defect can be primarily classified into three categories, viz. Vacancy, Interstitial, and Substitutional. Sometime Self-Interstitial defect is also considered another one category. However, in case of ionic crystals (ceramics), Schottky defect and Frenkel defect can occur, which are nothing but the combination of two different types of the above mentioned four basic types of point defects. The details of these point defects are discussed in the following section.
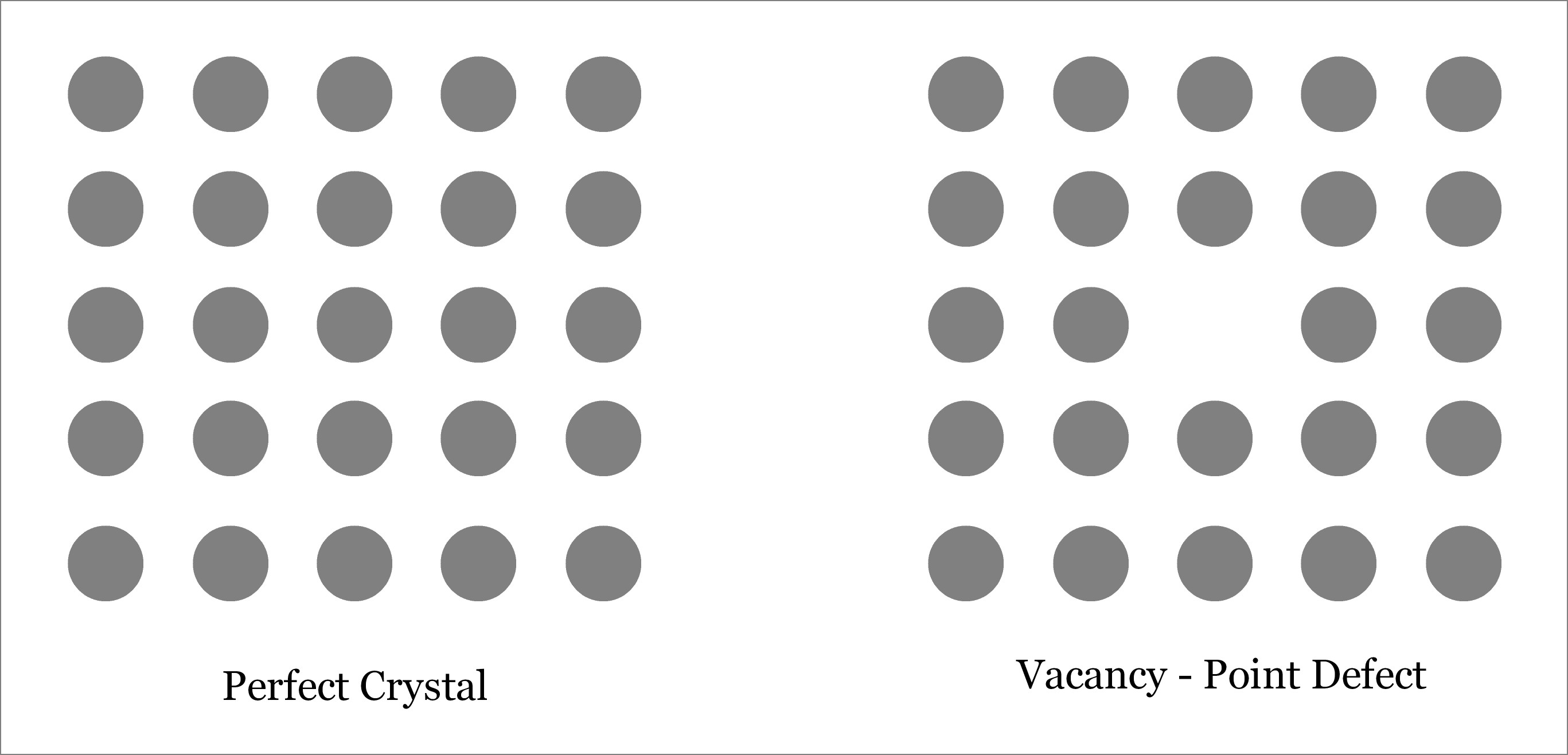
Vacancy – A Point Defect
A vacancy is produced when an atom is missing from its original lattice site. So vacancy creates an empty lattice site as depicted below. Like other point defects, vacancy is also a zero-dimensional defect. Vacancy defect puts the neighboring atoms under tension. Due to the reduction in number of atoms in the crystalline solid, vacancy defect results in reduction of the density. However, hardness of the solid may increase. The number of vacancies present within a crystalline solid depends exponentially with temperature, thus with increase in temperature of solid, number of vacancies also increases. You can learn more and solve problems regarding vacancy defect. Learn more about Vacancy defect.
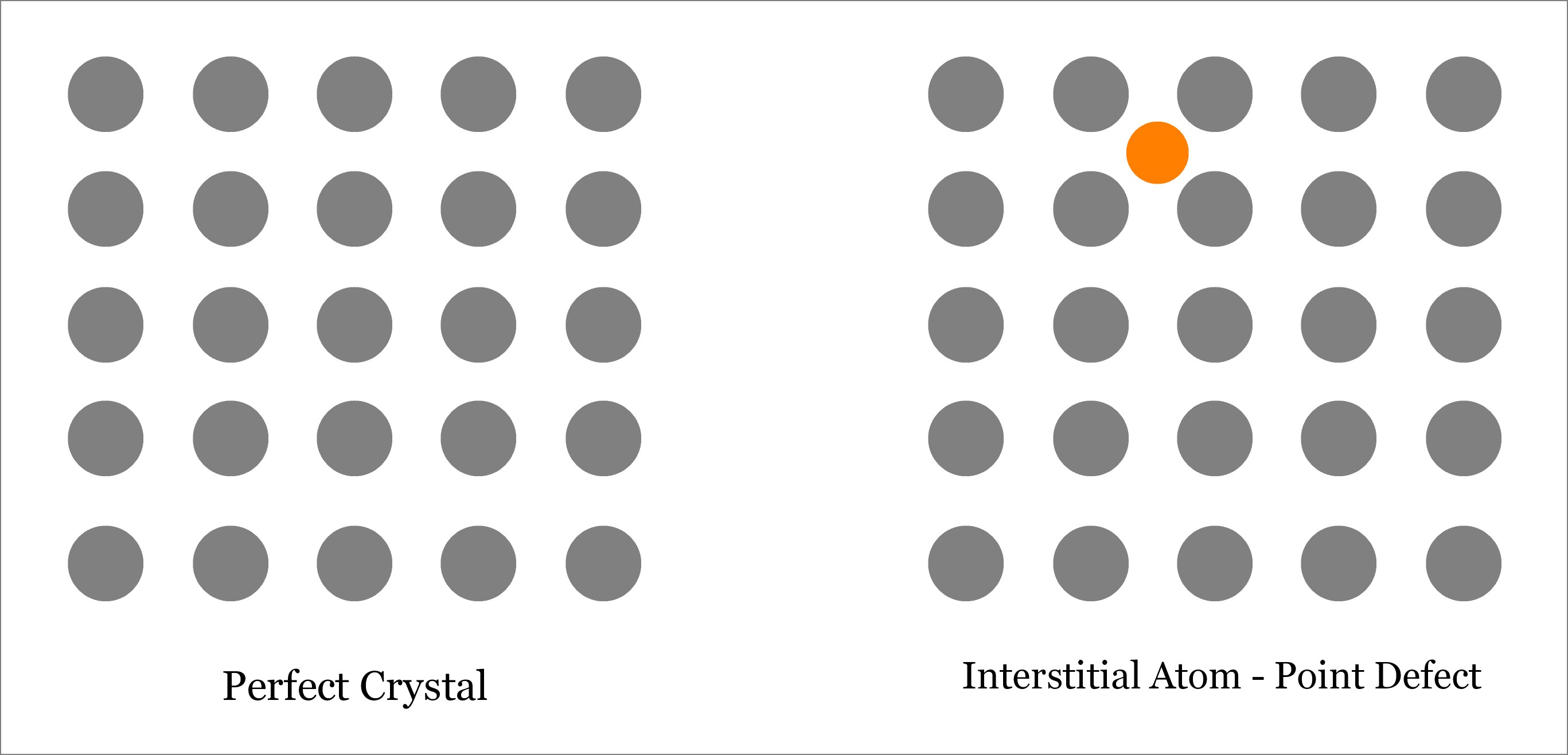
Interstitial – A Point Defect
An interstitial defect occurs when an atom takes the interstitial position of the lattice structure. This interstitial atom may be of the same crystal or of a foreign material. Accordingly, interstitial defect can be of two types:
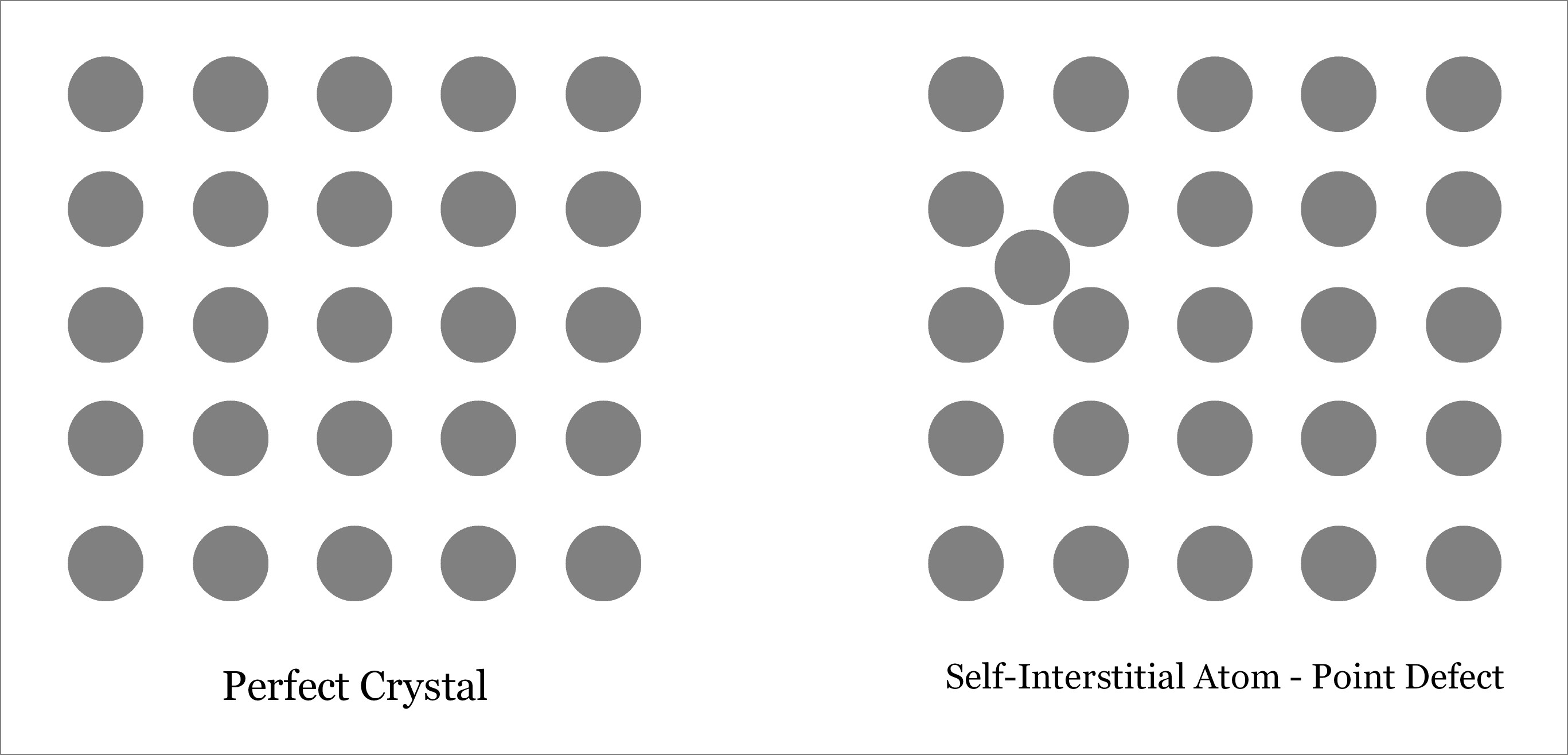
- Self-Interstitial Defect—occurs when atom of the same crystalline solid occupies the interstitial position leaving its original lattice site.
- Interstitial Defect—occurs when a foreign atom occupies the interstitial position.
Although extra atom occupies the empty interstitial space, the size of the atom is usually larger than that of the empty space. Thus the surrounding atoms are compressed and distorted. Presence of substantial number of interstitial atoms can change the mechanical and thermal properties of the solid. However, this is sometime beneficial, and thus interstitial defects can be applied in a controlled way to enhance various properties of the solid. For example, in steel production, carbon is added with iron. Learn more about Interstitial defect.
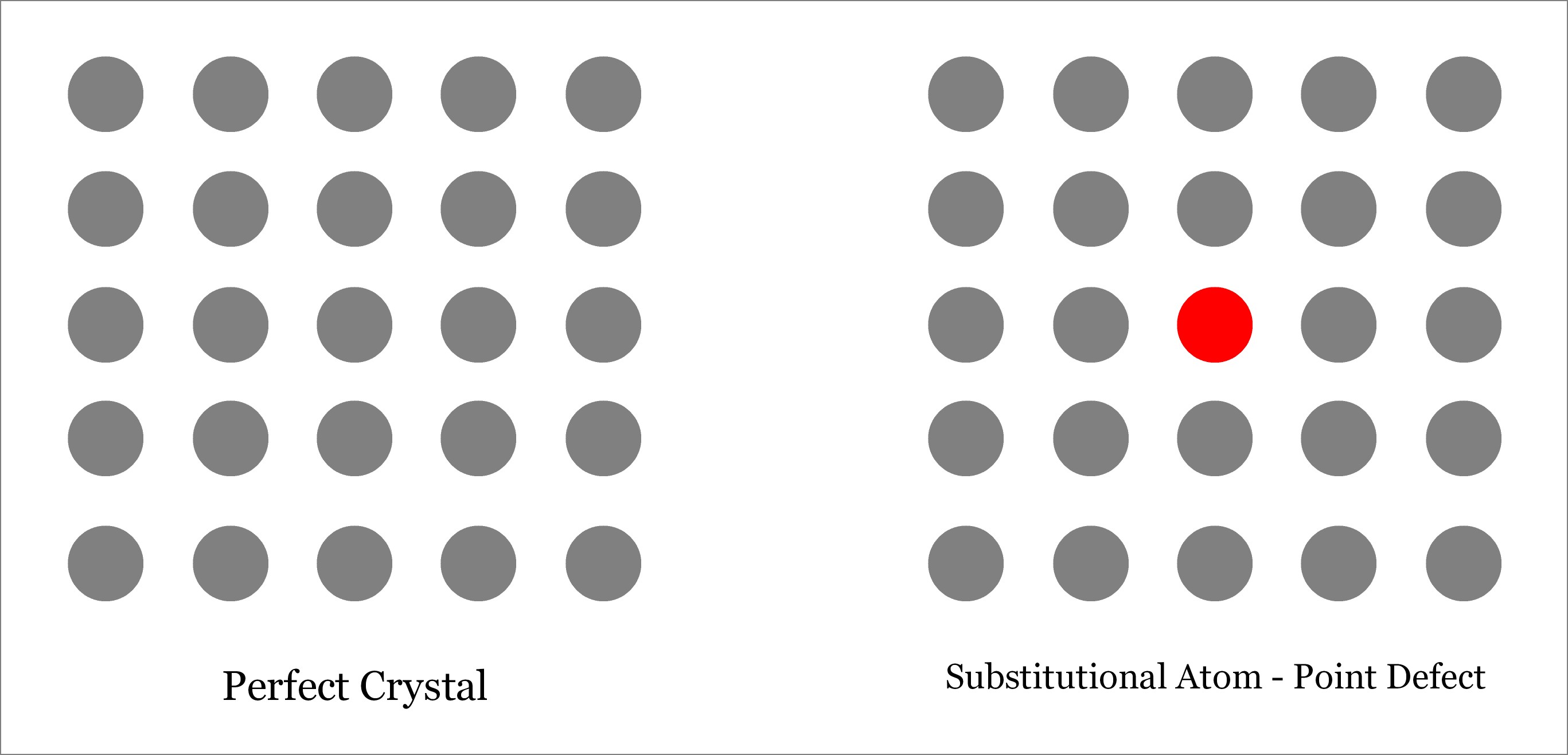
Substitutional – A Point Defect
Substitutional Defect occurs when the original atom in the lattice site of a crystalline solid is replaced by a different type of atom. Unlike interstitial defect, foreign atom should occupy the lattice site only and not the interstitial position, as depicted below. The foreign atom may be of same size or different (either larger or smaller). Depending on the size of the substituted foreign atom, the neighboring atoms may remain either in tension or in compression. Substitutional defects can be found in brass, where zinc atoms replace copper atoms. Learn more about Subctitutional defect.
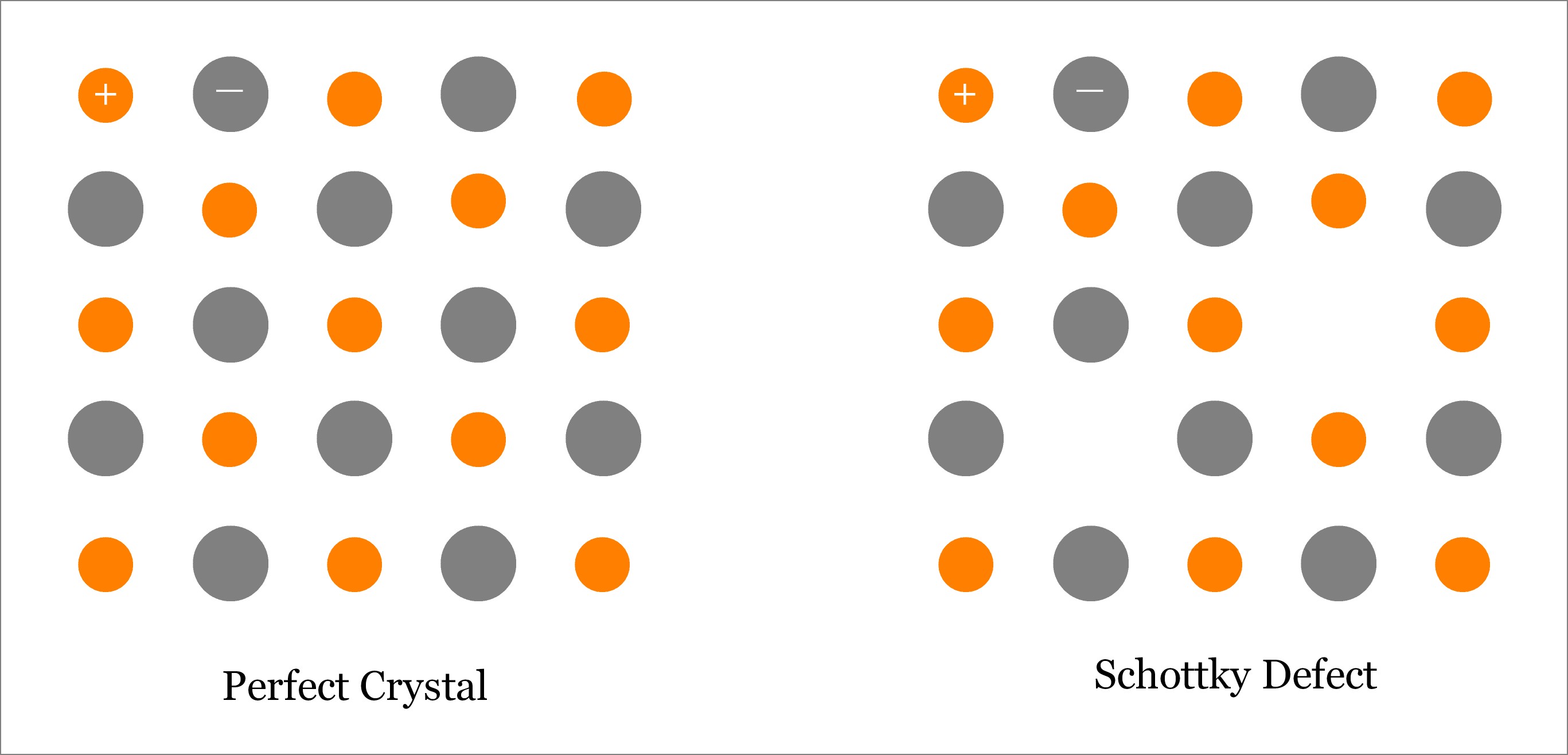
Schottky Defect – Point Defect in Ionic Crystal (Ceramic)
It is one type of Point Defect that occurs in ionic crystals (ceramics). Schottky defect occurs when oppositely charged atoms (cation and anion) leave their corresponding lattice sites and create a pair of Vacancy Defects. So, one Schottky defect leads to the formation of two vacancies. Since both cation and anion leave the lattice sites at the same time, so overall electrical neutrality of the crystal is maintained; however, density reduces because of the vacancies. Learn more about Schottky defect.
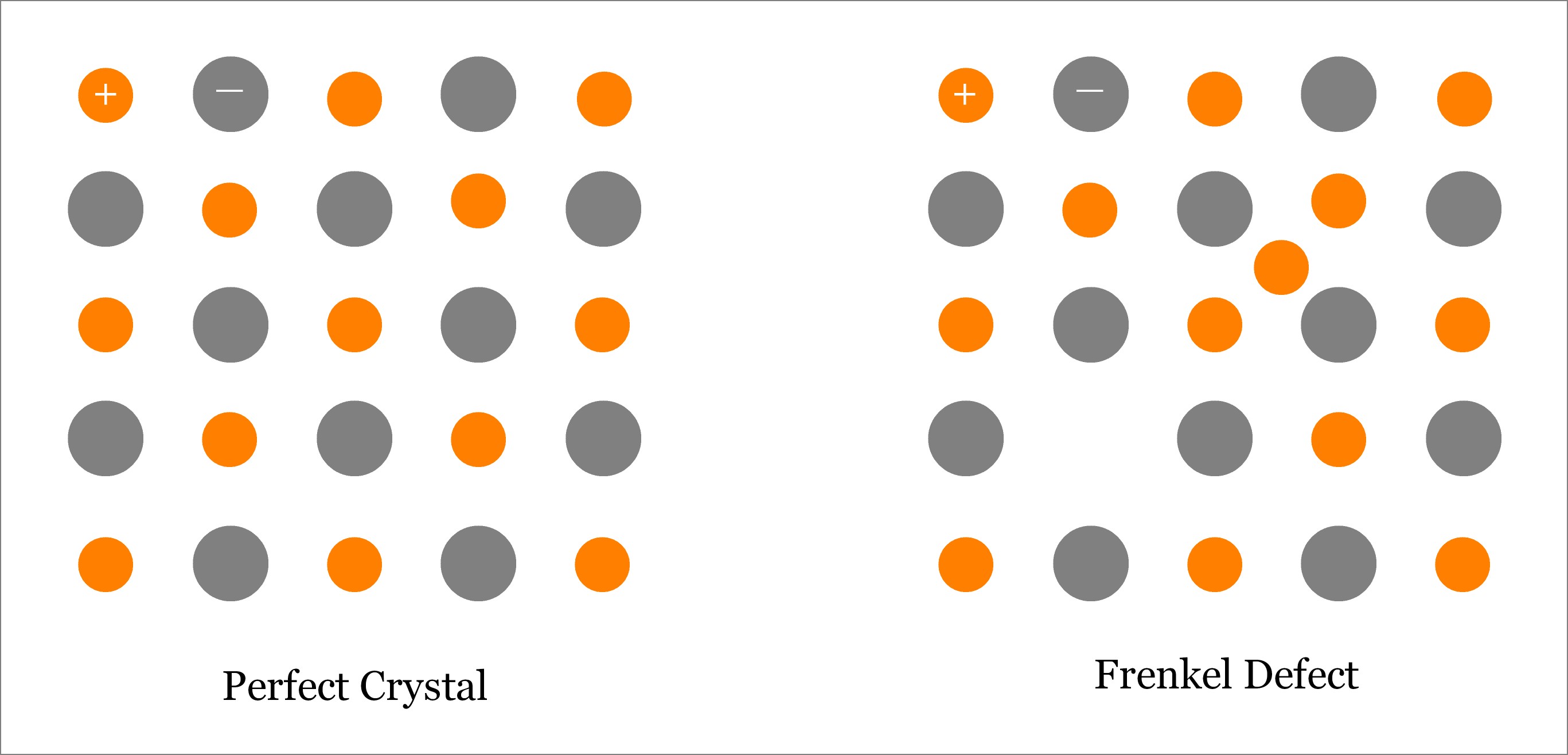
Frenkel Defect – Point Defect in Ionic Crystal (Ceramic)
Frenkel Defect is one type of Point Defect; in fact, it is a combination of both Vacancy and Interstitial type of point defects. Usually, this type of defect is observed in ionic solids, where size of anion is substantially larger than the size of cation. Basically, a Frenkel Defect is occurs when an atom (better to say ion, especially cation) leaves its original lattice site and occupies an interstitial position on the same crystal. Although both—Schottky and Frenkel defects occur in ionic materials, Frenkel defect occurs if size of anion is quite large as compared to that of the cation; whereas, Schottky defect occurs if difference in size between cation and anion is small. In Frenkel defect, only the smaller ion (cation) leaves its original lattice site; whereas, the anion remains in corresponding lattice sites. However, in Schottky defect, both cation and anion leave the solid crystal. Learn more about Frenkel Defect.